Clinical Trials and Efficacy

FABIOR Foam was studied as monotherapy in patients with moderate-to-severe acne
FABIOR Foam was studied in 2 randomized, double-blind, vehicle-controlled studies of 1485 patients aged 12 to 45 years1
- 51% of patients were female, 49% of patients were male
- 58% of patients were between the ages of 12 and 17
- 77% of patients were white, 15% of patients were black, 8% were race other than white or black
- At baseline, 80% of patients were assessed as moderate (IGA 3), and 20% of patients were assessed as severe (IGA 4)
Patients were randomized 1:1 to FABIOR Foam or vehicle foam, applied once daily for 12 weeks1
- While patients were required to treat the face, they were also permitted to treat the trunk
- Use of concomitant acne treatments was not permitted
Efficacy was evaluated using lesion counts and the 6-point Investigator’s Global Assessment (IGA) scale2
- Absolute change in lesion counts (total, inflammatory, and non-inflammatory) from baseline to week 12
- Treatment success, which was defined as an IGA score of "clear" (grade 0) or "almost clear" (grade 1) and at least a 2-grade improvement from the baseline score at week 12.
Clinical results achieved with FABIOR Foam: actual patients from clinical trials
Patient 1*
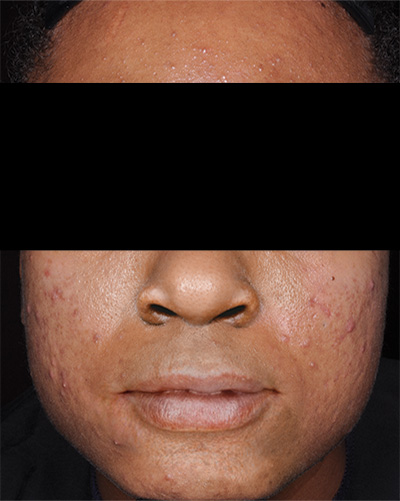
Baseline
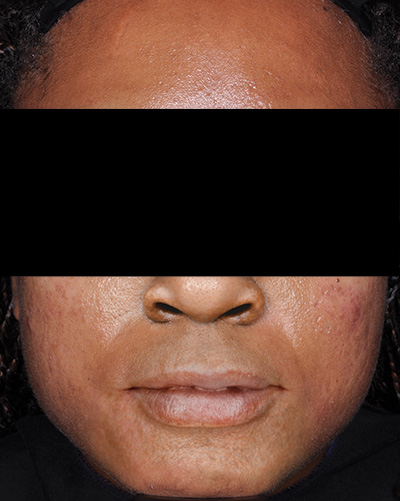
Week 12
Patient 2*
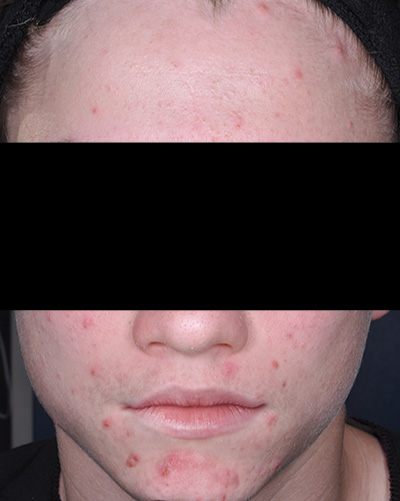
Baseline
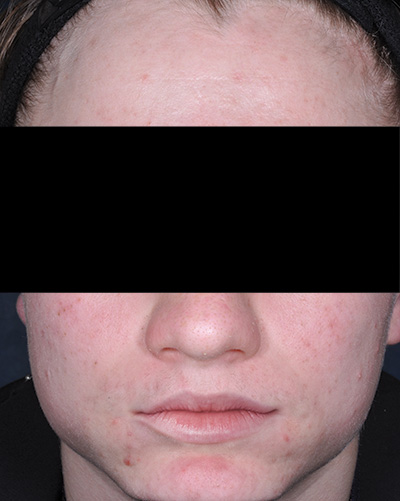
Week 12
*Actual patients from clinical trials whose facial features have been modified to protect their confidentiality. Representative of clinical trial efficacy. Individual results may vary.
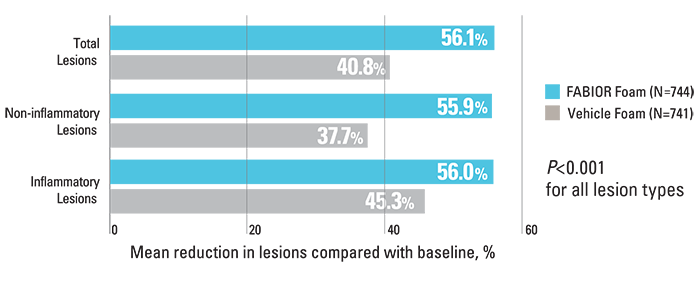
FABIOR Foam, used as monotherapy in moderate-to-severe acne patients, demonstrated a statistically significant reduction in total acne lesions, inflammatory lesions, and non-inflammatory lesions.2
Reduction in acne lesions at week 12 compared with baseline
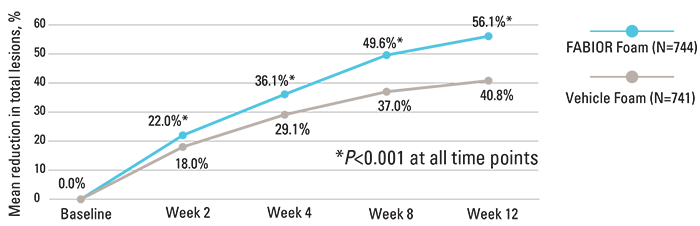
FABIOR Foam demonstrated statistically significant efficacy as early as week 2, with continued improvement for the duration of the study.
Reduction in total acne lesions over time2
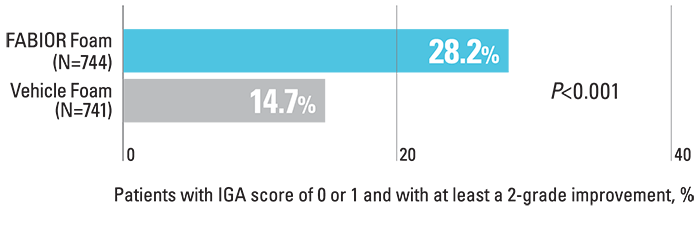
Significantly more patients achieved treatment success with FABIOR Foam compared with vehicle foam at week 12.
Percentage of patients achieving treatment success3
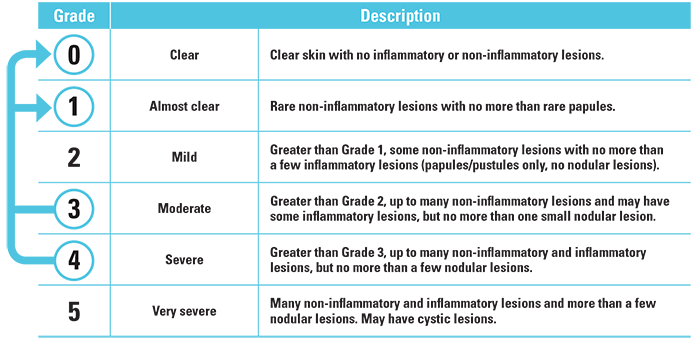
Treatment success was defined as an IGA score of “clear” (grade 0) or “almost clear” (grade 1) and at least a 2-grade improvement from the baseline score at week 12.
Investigator’s Global Assessment (IGA) Scale
References: 1. FABIOR Foam [package insert]. Greenville, NC: Mayne Pharma; 2016. 2. Stein Gold L, Jasper S. Tazarotene foam 0.1% for Acne Vulgaris: analysis of integrated efficacy and safety data by race. Presented at: Proceedings of the Summer Academy Meeting American Academy of Dermatology; August 15-19, 2012; Boston, MA. 3. Data on file; Greenville, NC: Mayne Pharma LLC.


